You’ve made up your mind.
You’re going to supplement your diet with more protein. Fantastic.
But as you browse online you are attacked by an endless list of protein powders.
Whey protein. Casein protein. Pea protein. Rice protein. Apple-pie protein.
Okay, the last one isn’t a real thing (yet). But there is definitely no shortage of exotically named protein supplements.
So what’s the best protein powder for muscle gain?
Well first off, let’s establish...
What Matters In Your Protein Powder
There are four key areas to consider when evaluating a protein powder—
The sections below provide an overview of each area.
If you want to skip to the comparison, you can go straight to the Protein Comparison Table.
Protein Percentage
This is the protein percentage by calorie.
For example, this nutrition label—
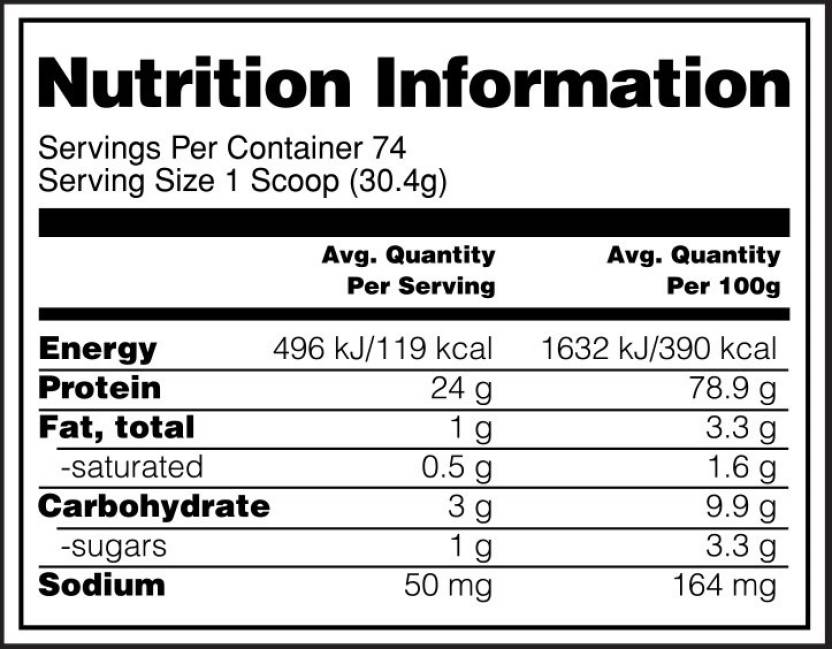
Example protein powder nutrition label.
Shows that the protein powder has approximately 81% protein by calories. That’s calculated via—
78.9 g of protein × 4 kcal per g protein ≈ 316 kcal
( 316 kcal / 390 kcal ) × 100 = 81%
(You can use this calorie and macro calculator to estimate how much protein you should eat each day)
Different protein powders have very different levels of protein content. From as low as 60% protein to above 95% protein, the range is huge.
The variation in protein percentage depends on two factors:
The Type Of Protein
The type of protein means either a completely different source of protein or the same source but refined to a different extent.
An example of a completely different source is whey protein vs wheat protein. Whey protein is derived from milk. Wheat protein is derived from wheat. These are fundamentally different sources of protein.
The process of extracting protein from milk is different from the process of extracting protein from wheat. Part of the final cost of the protein powder will depend on how costly it is to refine and isolate the protein from the source.
More refinement → More costly.
There is a point where it is no longer economical to further refine a protein source to get a higher percentage yield of protein. This is reflected in the final protein content of different sources of protein.
It’s why you see rice and hemp protein (generally) having lower protein content than whey protein. Extracting whey protein from milk is cheaper than extracting the equivalent amount of protein from rice or hemp.

Hemp protein is made from hemp seeds. Yes – weed.
An example of the same source refined to a different extent is Whey Protein Isolate vs Whey Protein Concentrate.
Both Whey Protein Isolate (WPI) and Whey Protein Concentrate (WPC) are derived from milk. The source of protein is the same.
The difference here is the refinement process. You get whey protein by pushing milk through a filter, removing the curd (that’s your casein protein). Let the whey protein dry, and you’re left with your WPC.
If you keep processing and refining your WPC – removing more lactose and fat – you get WPI.
WPI is just a more refined version of WPC.
As you would expect, removal of lactose and fat results in WPI having a higher protein content than WPC, regardless of the supplier.
The Brand Of Protein
The brand of a protein powder comes into play when you’re comparing the same protein from two different suppliers.
For example, Peter’s Protein and Steve’s Supplements both sell Whey Protein Concentrate (WPC).
But Peter’s WPC has 85% protein, while Steve’s WPC only has 70% protein.
This is a common occurrence: there is a large variation in protein content between suppliers, even for the same type of protein powder.
This variation depends on the amount of filler and flavouring they put into the protein, as well as their refinement process.
This can vary a lot. If you’ve compared the nutrition label of a few brands of WPC, you’ll know exactly what I mean.
This is why it’s important to look deeper than just the type of protein. The brand matters too – not all suppliers were made equal. The best protein powders often have significantly higher protein content than inferior products on the market.
Amino Acid Content
Amino acids are the building blocks of protein. They come in two forms:
- Unessential Amino Acids are amino acids naturally made by your body. 11 of the 20 amino acids are “unessential”.
- Essential Amino Acids (EAAs) are amino acids that aren’t made by your body, which instead need to be consumed as part of your diet. 9 of the 20 amino acids are “essential”.
3 of those 9 EAAs are categorised as Branched-Chain Amino Acids – or BCAAs for short. These have been found to be particularly important for building muscle.
Leucine is the most powerful BCAA, and the single most important nutrient for building muscle. There is a lot of research demonstrating the importance of leucine in muscle building.
By definition, EAAs need to be consumed as part of your diet. And if there were any part of your diet you would expect to get your EAAs, it would be your protein supplements.
Here’s the thing: the type and amount of amino acids will depend on the type of protein.
Not all protein was made equal. 10g of protein from eggs is going to have a completely different amino acid composition than 10g of protein from spinach.
As a visual example, the graph below compares the EAA content of whey protein vs rice protein. The data was taken from this study comparing whey protein to rice protein.
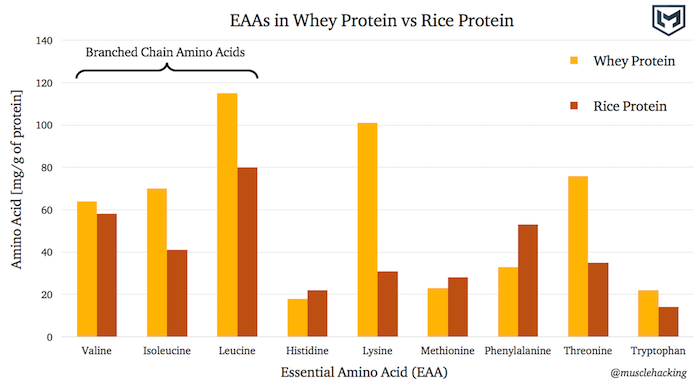
Essential Amino Acid Composition of Whey Protein vs Rice Protein
Whey protein is higher in all three BCAAs, and overall has a higher EAA content for 6 of the 9 EAAs.
4 of these 6 EAAs – Isoleucine, Leucine, Lysine, and Threonine – are a lot higher in whey protein than they are in rice protein.
This translates to more EAA bang for your buck per gram of whey protein versus rice protein. The source of the protein makes a difference.
Hence we have to consider more than just the pure protein content of a protein powder. The amino acid composition—particularly the BCAA and leucine content—should also be taken into account.
Speed Of Digestion
This is how long it takes for your protein to be digested.
An example of this is whey protein vs casein protein. Both of these proteins are derived from milk – milk protein being 20% whey protein and 80% casein protein.
Whey protein is digested rapidly – reaching peaking amino acid levels after an hour, and taking about 4 hours to fully digest.
Casein protein takes longer – the amino acid levels don’t peak as high, but are sustained for over 7 hours.
This doesn’t matter as much as most supplement companies would have you believe.
This is because it’s a mistake to consider the consumption of your protein supplements in a vacuum.
It’s true that casein protein won’t pass through your body as quickly as whey protein. But this neglects the fact that you’ll have:
- Protein flowing through your veins from other sources
- Other food slowing the digestion of your protein
For instance, many recommend that you consume casein protein – as opposed to whey protein – before bed. The logic is to allow the protein to digest slowly while you sleep.
In a vacuum, this sounds reasonable.
But you don’t live in a vacuum – you had steak and veggies for dinner. Or a similar mixed meal of fats, carbohydrates, and protein.
So there will already be protein flowing through your body from that meal. This makes the speed of digestion a non-factor.
This is reflected in the literature, with the majority of studies finding no significant difference between whey protein and casein protein’s ability to enhance body composition.
That means that the “slow digesting” nature of a protein isn’t an important factor of consideration.
Well then, what about the other side of the coin: digesting really fast.
Whey hydrolysate jumps to mind as the fan-favourite example. Whey hydrolysate is taking whey protein refinement to the next level, breaking down some of the amino acid bonds by exposing them to heat, acids, or enzymes.
Effectively whey hydrolysate is whey protein that has been broken down into smaller molecules for quicker absorption. This pre-digestion makes hydrolysed proteins more rapidly absorbed than whey concentrates or isolates.
The story behind their benefit sounds believable: this lightning-fast digestion allows you to deliver a muscle-building dose of protein straight to your biceps post-workout.
Sounds sexy right?
Slight issue though – it doesn’t work like that.
For two main reasons:
- The post-workout anabolic window has been revealed to be irrelevant to most lifters.
- Whey hydrolysate has been shown to be less effective in building muscle.
That’s right.
In a 2013 study, athletes consumed either whey protein concentrate (WPC), whey protein hydrolysate (WPH), or pure sugar.
The scientists found that WPH decreased muscle damage more than either WPC or the sugar.
This sounds like something we would want, but it’s not.
Since muscle damage is actually necessary for muscle growth to occur, WPH had a negative effect on body composition.
The study found that WPH resulted in 65% less muscle gain than the WPC.
And if that wasn’t bad enough: the WPH resulted in fat gain instead of fat loss. The WPC and sugar didn’t.
So the WPH was found to be more fattening than a shake of sugar.
Don’t pay more for less. More research needs to be done on whey protein hydrolysate before anyone can promote it as “superior” for muscle building. For now, it’s all hype.
Overall this means that your protein’s speed of digestion – whether it be super fast or super slow – is not a good reason to pay more.
Hence the speed of digestion has not been included in the Protein Comparison Table.
Price And Taste
Unless you’re a billionaire without taste buds, price and taste matter.
In an ideal world they wouldn’t – the best protein powder would be the healthiest and most pure, irrespective of its taste or price tag. That’s not the world we live on though (not to mention that I like my taste buds. I’ll take the cash though).
Thankfully in the realm of protein powder, you don’t need to break the bank to afford effective protein supplements.
It’s often the opposite: many of the more expensive forms of protein are actually less effective.
(same goes with many of the products in a good muscle building supplement stack).
This higher cost is typically the result of large advertising campaigns, and the ridiculous refinement processes required to extract the protein from this week’s trendy superfood.
So don’t make the mistake of thinking more expensive = more effective. It isn’t.
Taste varies a lot depending on the type of protein and the brand of protein.
For the type of protein, milk-derived protein powders tend to taste the best.
But you’ll see the largest variation in taste between different brands of protein.
The quality and quantity of sweetener(s) supplement companies use result in very different tasting protein. Even when they’re the exact same type of protein.
Taste-wise, the brand makes all the difference.
So before you order 10kg of protein powder from your chosen supplier, make sure you check the reviews online. If the company sells sample packs, order one of those first before you commit to a bulk purchase.
Protein Comparison Table
The table below rates each protein powder on five metrics: BCAA content, leucine content, protein content, price, and taste.
References are included below.
| Protein Source | BCAA | Leucine | Protein | Price | Taste |
|---|---|---|---|---|---|
| Whey Concentrate † | 26% | 14% | 75%+ | Low | Very good |
| Whey Isolate | 26% | 14% | 90%+ | Medium | Good |
| Whey Hydrolysateˆ | 26% | 14% | 94%+ | High | Medium |
| Casein | 21% | 9% | 85%+ | Medium | Good |
| Milk ♠ | 21% | 10% | 85%+ | Medium | Good |
| Egg | 20% | 8.5% | 90%+ | High | Poor |
| Beef Protein Isolate | 8% | 3% | 95%+ | High | Poor |
| Soy Isolate | 18% | 8% | 85%+ | Low | Medium |
| Wheat | 15% | 7% | 94%+ | High | Medium |
| Pea | 18% | 8.5% | 80%+ | Low | Medium |
| Rice | 18% | 8% | 60%+ | Low | Poor |
| Hemp | 16% | 7% | 60%+ | High | Medium |
There are many other suitable alternatives. Just be sure to check the percentage of protein per serving and that you enjoy the taste (in that order).
My detailed list of supplements that support muscle growth is available here.
Note: Speed of digestion has been omitted as its effect is negligible (as discussed). Remember that Price and Taste are referring to the protein in powder form. Normal eggs are cheap and delicious – but egg protein powder is expensive and gross.
† There is a large variation in the protein percentage of Whey Concentrate, although it is typically above 75%.
♠ Milk protein is 80% casein protein and 20% whey protein.
ˆ The incredibly fast digestion of Whey Hydrolysate may negatively impact muscle building. See discussion in Speed Of Digestion.
That’s it for this post. If you found it useful, consider sharing it on Facebook or Twitter.
Notes for Nerds
On BCAA and Leucine Content
Different studies report slightly different values for the amino acid profile of various proteins. Regardless, the relative differences between proteins (e.g. leucine in whey protein vs leucine in rice protein) remain largely the same.
On Slow vs Fast Digesting Protein
While there are a few studies that show statistically significant differences in muscle gain when comparing slow versus fast digesting protein, these are the exception, not the rule.
These studies use unrealistic experimental conditions, such as fasting for 12+ hours before the workout or consuming only the protein supplement post-workout.
Many of these studies are funded by industries that have a vested interest in there being a difference. Even under these ideal conditions for differences to reveal themselves, the majority of studies show no difference.
Hence the onus is on further research to strengthen the argument that the speed of digestion of a protein has a practical application in muscle building for the average gym-goer.
Note that by ‘average’ I mean people who aren’t working out 2+ times per day or fasting for long periods of time pre or post workout.
References
Importance Of Leucine And BCAAs
- B. Pennings, B. Groen, A. de Lange, A. P. Gijsen, A. H. Zorenc, J. M. G. Senden, and L. J. C. van Loon, “Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men,” AJP: Endocrinology and Metabolism, vol. 302, pp. E992–E999, apr 2012.
- T. A. Churchward-Venne, N. A. Burd, C. J. Mitchell, D. W. West, A. Philp, G. R. Marcotte, S. K. Baker, K. Baar, and S. M. Phillips, “Supplementation of a suboptimal protein dose with leucine or essential amino acids: Effects on myofibrillar protein synthesis at rest and following resistance exercise in men,” Journal of Physiology, vol. 590, no. 11, pp. 2751–2765, 2012.
- M. G. Buse and S. S. Reid, “Leucine. A possible regulator of protein turnover in muscle,” Journal of Clinical Investigation, vol. 56, pp. 1250–1261, nov 1975.
- S. O. Hong and D. K. Layman, “Effects of leucine on in vitro protein synthesis and degradation in rat skeletal muscles.,” The Journal of Nutrition, vol. 114, pp. 1204–12, jul 1984.
- J. C. Anthony, T. G. Anthony, S. R. Kimball, and L. S. Jefferson, “Signaling Pathways Involved in Translational Control of Protein Synthesis in Skeletal Muscle by Leucine,” The Journal of Nutrition, vol. 131, pp. 856S–860S, apr 2001.
- X. J. Zhang, D. L. Chinkes, and R. R. Wolfe, “Leucine supplementation has an anabolic effect on proteins in rabbit skin wound and muscle,” The Journal of Nutrition, vol. 134, pp. 3313–3318, dec 2004.
- D. J. Wilkinson, S. S. Bukhari, B. E. Phillips, M. C. Limb, J. Cegielski, M. S. Brook, D. Rankin, W. K. Mitchell, H. Kobayashi, J. P. Williams, J. Lund, P. L. Greenhaff, K. Smith, and P. J. Atherton, “Effects of leucine-enriched essential amino acid and whey protein bolus dosing upon skeletal muscle protein synthesis at rest and after exercise in older women,” Clinical Nutrition, pp. 1–11, 2017.
- P. J. Atherton, V. Kumar, A. L. Selby, D. Rankin, W. Hildebrandt, B. E. Phillips, J. P. Williams, N. Hiscock, and K. Smith, “Enriching a protein drink with leucine augments muscle protein synthesis after resistance exercise in young and older men,” Clinical Nutrition, vol. 36, no. 3, pp. 888–895, 2017.
- J. M. Dickinson, D. M. Gundermann, D. K. Walker, P. T. Reidy, M. S. Borack, M. J. Drum, M. Arora, E. Volpi, and B. B. Rasmussen, “Leucine-Enriched Amino Acid Ingestion after Resistance Exercise Prolongs Myofibrillar Protein Synthesis and Amino Acid Transporter Expression in Older Men 1 - 3,” The Journal of Nutrition, vol. 144, pp. 1694–1702, 2014.
- P. J. Atherton, K. Smith, T. Etheridge, D. Rankin, and M. J. Rennie, “Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells,” Amino Acids, vol. 38, no. 5, pp. 1533–1539, 2010.
- J. C. Anthony, T. G. Anthony, and D. K. Layman, “Leucine supplementation enhances skeletal muscle recovery in rats following exercise.,” The Journal of Nutrition, vol. 129, pp. 1102–6, jun 1999.
- R. Koopman, “Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects,” AJP: Endocrinology and Metabolism, vol. 288, pp. E645–E653, apr 2004.
- Y. C. Luiking, N. E. Deutz, R. G. Memelink, S. Verlaan, and R. R. Wolfe, “Postprandial muscle protein synthesis is higher after a high whey protein, leucine-enriched supplement than after a dairy-like product in healthy older people: A randomized controlled trial,” Nutrition Journal, vol. 13, p. 9, dec 2014.
- S. R. Kimball and L. S. Jefferson, “Regulation of protein synthesis by branched-chain amino acids.,” Current Opinion in Clinical Nutrition and Metabolic Care, vol. 4, pp. 39–43, jan 2001.
- J. B. Li and L. S. Jefferson, “Influence of amino acid availability on protein turnover in perfused skeletal muscle,” Biochemica et Biophysica Acta, vol. 544, pp. 351–359, 1978.
- B. Cambell, R. B. Kreider, T. Ziegenfuss, P. L. Bounty, M. Roberts, D. Burke, J. Landis, H. Lopez, and J. Antonio, “International Society of Sports Nutrition position stand: protein and exercise,” Journal of International Society of Sports Nutrition, vol. 4, no. 23, pp. 1–5, 2007.
- D. J. Millward, D. K. Layman, D. Tomé, and G. Schaafsma, “Protein quality assessment: impact of expanding understanding of protein and amino acid needs for optimal health,” The American Journal of Clinical Nutrition, vol. 87, pp. 1576–81, may 2008.
Whey Protein vs Casein Protein
- S. L. Urbina, A. White, J. Shaw, C. Wilborn, and B. Brabham, “The effects of post-exercise whey vs. casein protein ingestion on muscular strength, muscular endurance, and body composition in older women (50-70 years of age),” Journal of the International Society of Sports Nutrition, vol. 8, no. Suppl 1, p. P27, 2011.
- C. D. Wilborn, L. W. Taylor, J. Outlaw, L. Williams, B. Campbell, C. A. Foster, A. Smith-Ryan, S. Urbina, and S. Hayward, “The effects of pre- and post-exercise whey vs. casein protein consumption on body composition and performance measures in collegiate female athletes,” Journal of Sports Science and Medicine, vol. 12, no. 1, pp. 74–79, 2013.
Protein Timing
- A. A. Aragon and B. J. Schoenfeld, “Nutrient timing revisited: Is there a post-exercise anabolic window?,” Journal of the International Society of Sports Nutrition, vol. 10, 2013.
Hydrolysed Whey
- P. C. Lollo, J. Amaya-Farfan, I. C. Faria, J. V. Salgado, M. P. Chacon-Mikahil, A. G. Cruz, C. A. Oliveira, P. C. Montagner, and M. Arruda, “Hydrolysed whey protein reduces muscle damage markers in Brazilian elite soccer players compared with whey protein and maltodextrin. A twelve-week in-championship intervention,” International Dairy Journal, vol. 34, no. 1, pp. 19–24, 2014.
- C. M. Lockwood, M. D. Roberts, V. J. Dalbo, A. E. Smith-Ryan, K. L. Kendall, J. R. Moon, and J. R. Stout, “Effects of Hydrolyzed Whey versus Other Whey Protein Supplements on the Physiological Response to 8 Weeks of Resistance Exercise in College-Aged Males,” Journal of the American College of Nutrition, vol. 36, no. 1, pp. 16–27, 2017.
BCAA And Leucine Content
- D. K. Layman and J. I. Baum, “Dietary protein impact on glycemic control during weight loss.,” The Journal of Nutrition, vol. 134, pp. 968S–73S, oct 2004.
- C. C. Almeida, T. S. Alvares, M. P. Costa, and C. A. Conte-Junior, “Protein and Amino Acid Profiles of Different Whey Protein Supplements,” Journal of Dietary Supplements, vol. 13, no. 3, pp. 313–323, 2016.
- J. Overduin, L. Guérin-Deremaux, D. Wils, and T. T. Lambers, “NUTRALYS® pea protein: Characterization of in vitro gastric digestion and in vivo gastrointestinal peptide responses relevant to satiety,” Food and Nutrition Research, vol. 59, pp. 1–9, 2015.
- V. R. Young and P. L. Pellett, “Plant proteins in relation to human protein and amino acid nutrition,” in The American Journal of Clinical Nutrition, vol. 59, pp. 1203S–1212S, Oxford University Press, may 1994.
- P. E. Miller, D. D. Alexander, and V. Perez, “Effects of Whey Protein and Resistance Exercise on Body Composition: A Meta-Analysis of Randomized Controlled Trials,” Journal of the American College of Nutrition, vol. 33, no. 2, pp. 163–175, 2014.
- C. V. Morr and E. Y. Ha, “Whey Protein Concentrates and Isolates: Processing and Functional Properties,” Critical Reviews in Food Science and Nutrition, vol. 33, pp. 431–476, jan 1993.
- S. Sindayikengera and W. Xia, “Nutritional evaluation of caseins and whey proteins and their hydrolysates from Protamex,” Journal of Zhejiang University Science B, vol. 7, no. 2, pp. 90–98, 2006.
- S. Rafiq, N. Huma, I. Pasha, A. Sameen, O. Mukhtar, and M. I. Khan, “Chemical composition, nitrogen fractions and amino acids profile of milk from different animal species,” Asian-Australasian Journal of Animal Sciences, vol. 29, no. 7, pp. 1022–1028, 2016.
- W. G. Gordon, W. F. Semmett, and M. Bender, “Amino Acid Composition of γ-Casein,” Journal of the American Chemical Society, vol. 75, no. 3, pp. 1678–1679, 1953.
- B. H. Lauer and B. E. Baker, “Amino acid composition of casein isolated from the milks of different species.,” 1977.
- N. Ganguli, R. Prabhakaran, and K. Iya, “Composition of the Caseins of Buffalo and Cow Milk,” Journal of Dairy Science, vol. 47, no. 1, pp. 13–18, 1964.
- B. Y. W. G. Gordon and W. F. Semmett, “Amino Acid Composition of α-Casein and β-Casein,” Journal of the American Chemical Society, vol. V, no. 3, 1949.
- D. Kalman, “Amino Acid Composition of an Organic Brown Rice Protein Concentrate and Isolate Compared to Soy and Whey Concentrates and Isolates,” Foods, vol. 3, no. 3, pp. 394–402, 2014.
- J. M. Joy, R. P. Lowery, J. M. Wilson, M. Purpura, E. O. De Souza, S. M. Wilson, D. S. Kalman, J. E. Dudeck, and R. Jäger, “The effects of 8 weeks of whey or rice protein supplementation on body composition and exercise performance,” Nutrition Journal, vol. 12, no. 1, pp. 1–7, 2013.
- J. C. Callaway, “Hempseed as a nutritional resource: An overview,” Euphytica, vol. 140, no. 1-2, pp. 65–72, 2004.
- X. S. Wang, C. H. Tang, X. Q. Yang, and W. R. Gao, “Characterization, amino acid composition and in vitro digestibility of hemp (Cannabis sativa L.) proteins,” Food Chemistry, vol. 107, no. 1, pp. 11–18, 2008.
- J. D. House, J. Neufeld, and G. Leson, “Evaluating the quality of protein from hemp seed (Cannabis sativa L.) products through the use of the protein digestibility-corrected amino acid score method.,” Journal of Agricultural and Food Chemistry, vol. 58, no. 22, pp. 11801–11807, 2010.
- C. J. Detzel, B. W. Petschow, N. Johnson, and E. M. Weaver, “Comparison of the Amino Acid and Peptide Composition and Postprandial Response of Beef, Chicken, and Whey Protein Nutritional Preparations,” Functional Foods in Health and Disease, vol. 6, no. 10, pp. 612–626, 2016.
